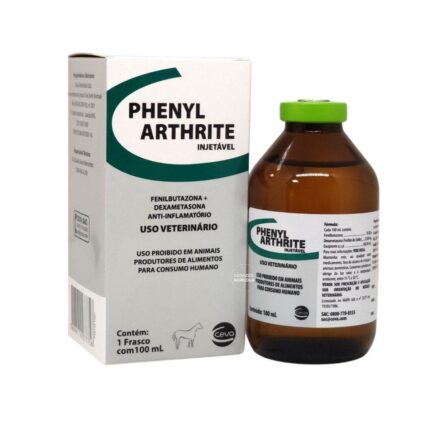
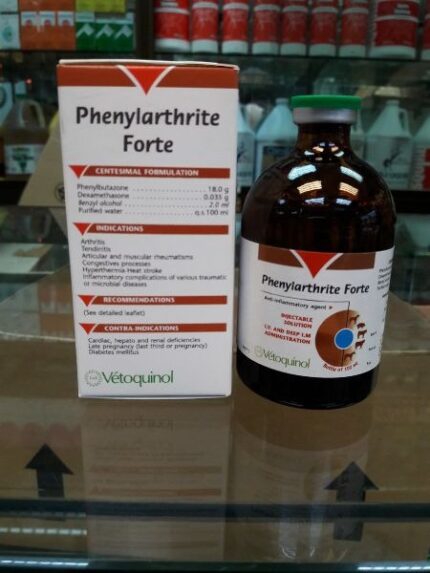
Phenylarthrite
$50.00 Original price was: $50.00.$45.00Current price is: $45.00.
Phenylarthrite with dexamethasone that enhances the anti-inflammatory, antipyretic and analgesic effect of the product.Combination of that enhances the anti-inflammatory, antipyretic and analgesic effect of the product.
Presentation: 100mL bottles.
Slow intravenous route
Application every 24 or 48 hours as follows:
Adult horses: 3 to 4 mL / 100 kg body mass.
Foals: 1 to 2 mL / 50 kg body mass.
Description
Phenylarthrite 100 ml phenylbutazone bute is used for the treatment of inflammation, pain and fever, particularl in the case of muscoskeletal disorders, congestive processes or inflammatory complications following traumatic of infectious affections.
Composition
Phenylbutazone 200 mg/ml
Indication
Dosage and administration
Arthritis, tendonitis, joint- and muscle rheumatism, hyperthermia, inflammations caused by trauma or bacteria.Dosage and administration
Horses: I.V.: inject slowly 20 ml daily or every other day, depending on the severity of the disorder.
I.M.: inject deeply 20 to 30 ml on day 1.
20 ml on day 2.
10 ml the following 1 to 4 days.
If necessary a second series of injections may be administered after 1 to 2 weeks.
Do not exceed the recommended dosage. If there is no improvement after 6 days discontinue treatment.Precautions
Use strict sterile conditions.Contraindications
Heart-, liver and kidney insufficiency.Withholding period
Do not use in animals intended for food.Warnings
It is recommended to decrease the dose or to discontinue treatment in animals with weak veins or with coagulation problems.Storage
Store at 15 – 25°C.
Keep out of reach and sight of children.Presentation
100 ml bottle
Description
Phenylarthrite 100 ml phenylbutazone bute is used for the treatment of inflammation, pain and fever, particularl in the case of muscoskeletal disorders, congestive processes or inflammatory complications following traumatic of infectious affections.
Composition
Phenylbutazone 200 mg/ml
Indication
Dosage and administration
Arthritis, tendonitis, joint- and muscle rheumatism, hyperthermia, inflammations caused by trauma or bacteria.Dosage and administration
Horses: I.V.: inject slowly 20 ml daily or every other day, depending on the severity of the disorder.
I.M.: inject deeply 20 to 30 ml on day 1.
20 ml on day 2.
10 ml the following 1 to 4 days.
If necessary a second series of injections may be administered after 1 to 2 weeks.
Do not exceed the recommended dosage. If there is no improvement after 6 days discontinue treatment.Precautions
Use strict sterile conditions.Contraindications
Heart-, liver and kidney insufficiency.Withholding period
Do not use in animals intended for food.Warnings
It is recommended to decrease the dose or to discontinue treatment in animals with weak veins or with coagulation problems.Storage
Store at 15 – 25°C.
Keep out of reach and sight of children.Presentationshia


At BioHoofMed, we are committed to delivering your equine care products quickly, safely, and with the highest level of professionalism. Every order is carefully processed, packaged, and shipped using trusted carriers to ensure your items arrive in perfect condition. We offer nationwide delivery throughout the United States, as well as reliable international shipping for customers around the world.
Our streamlined logistics system provides transparent order tracking, predictable delivery times, and timely updates from dispatch to arrival. Whether you’re ordering a single product or stocking up for your barn, we ensure a smooth, worry-free experience from checkout to delivery. Your horse’s health is our priority, and we work hard to make sure your products get to you exactly when you need them.






Reviews
There are no reviews yet.